Project Structure
The project work-plan is structured into four main strands, regular milestones ensure progress and usability in real-world settings.
1. Ecosystem Conception, defining ethical relationships, incentivization, and architecture.
2. Platform Infrastructure, establishing interoperable systems aligned with EHDS.
3. Platform Implementation, delivering tools for AI services and governance.
4. Pilot Data (Re)use Cases, validating the ecosystem via genomic data pilots.
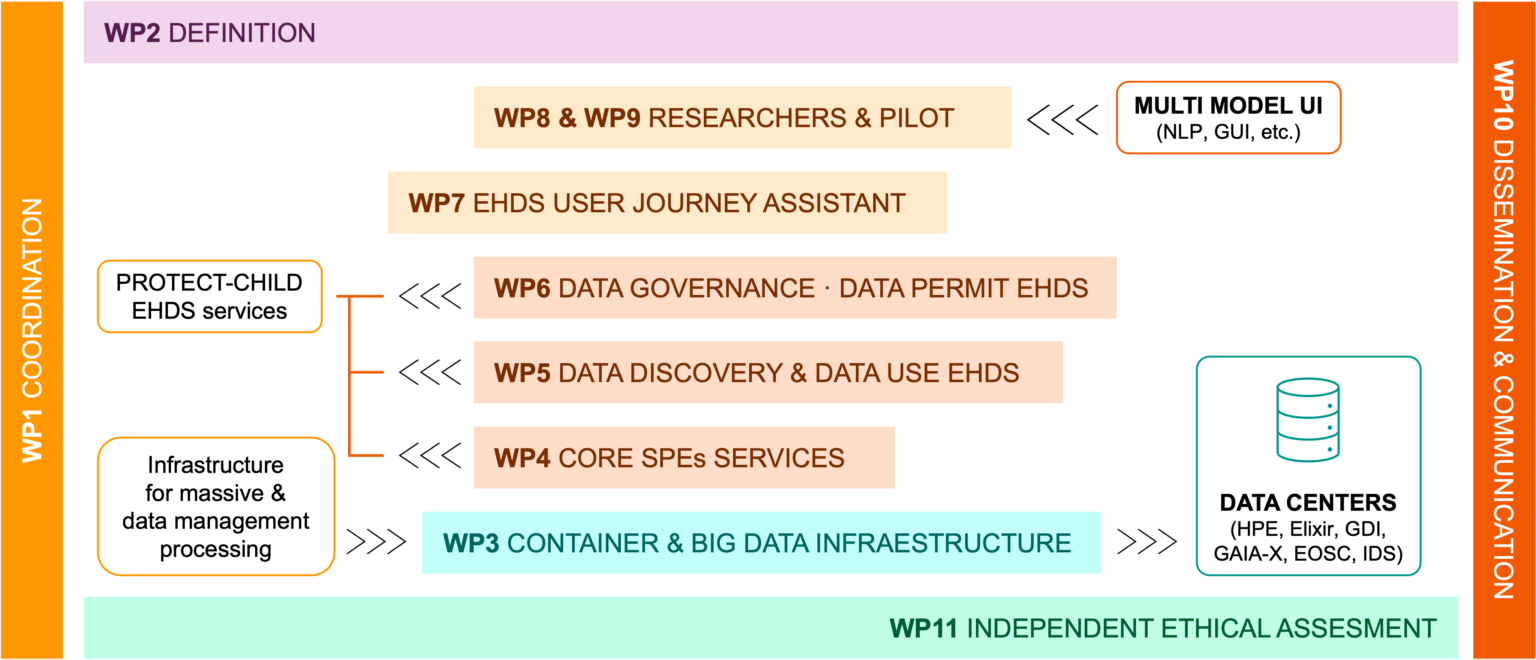
Summary of Work Packages
Work package WP1 – Coordination
Integrating genomic and clinical data from six European TransplantChild ERN hospitals addresses the scarcity of data and diverse responses in pediatric liver and kidney transplants.
Work package WP2 – Codesign and multi-stakeholders’ requirements
Co-create an ethical and legal framework, interoperable data models, and a federated platform architecture that enables privacy-preserving data processing, enhances data quality, and supports secure cross-border genomic and health data sharing within the EHDS framework.
Work package WP3 – Big Data Infrastructure for massive data management
Develop a high-availability infrastructure integrating public and private systems for genomic data storage, high-performance computing, and secure data management, ensuring compliance with security and interoperability standards.
Work package WP4 – PROTECT-CHILD Core EHDS services
Develop and integrate services for data discovery, governance, preparation, and use, ensuring compliance with FHIR and OMOP standards, and optimizing deployment and management with DevOps/MLOps tools.
Work package WP5 – Data discovery and data use framework
Provide data discovery and metadata services, privacy-preserving OLAP functionalities, and AI-driven genomic analysis, including federated learning, NLP tools for multilingual clinical data structuring, and quantum-secure multi-party computation to enhance data security, scalability, and efficiency.
Work package WP6 – Data Governance and ethical framework implementation
Develop Authentication, Authorization, and Audit (AAA) services, ethical review frameworks, and dynamic consent management systems to streamline data sharing and ensure compliance with EHDS regulations.
Work package WP7 – EHDS User Journey Assistant
Develop user-friendly assistants and dashboards to guide users through data discovery, permit management, and data use, ensuring privacy-preserving analysis and integration of genomic analysis within EHDS standards.
Work package WP8 – Pilot studies preparation and set-up
Establish clinical and technical setups to collect, annotate, and integrate clinical and genomic data for pilot studies, ensuring data harmonization, informed consent, and genomic risk analysis within the EHDS and GDI infrastructure.
Work package WP9 – Genome sequencing and analysis of PROTECT-CHILD pilot use cases
Perform genomic data collection and analysis for liver and kidney transplant recipients, integrating clinical data with genomic analysis for predictive modeling of immune responses and infections, assessing the effectiveness of PROTECT-CHILD tools in clinical settings.
Work package WP10 – Communication, dissemination and exploitation of results
Ensure the effective communication, dissemination, and exploitation of results to engage stakeholders and maximize impact and sustainability, including scientific dissemination, development of communication materials, and defining exploitation strategies.
Work package WP11 – Ethical, Legal and Social Issues Assessment
Ensure continuous ethical, legal, and social scrutiny of project activities, providing guidance on patient consent, project protocols, and managing ad hoc ethical, legal, and social issues in compliance with national and international regulatory standards.



